SURFACTANTS
Surfactants have a desired property: they dilute fats in water by dispersing them. Thus, they make it possible to modify the tension between two surfaces.
This property makes them essential in many industrial applications and also in everyday life. For example, mayonnaise is a stable emulsion of water and oil thanks to the surfactant contained in the egg yolk!
They therefore have an affinity for both oil and water. This unique, remarkable property enables them to solubilise two initially immiscible phases.
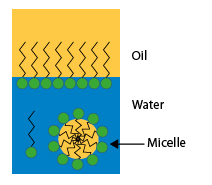
Dissolved in water, the surfactants gather at the interfaces, orienting themselves so that the hydrophilic part of their molecule is directed towards the water and the hydrophobic part towards the oily phase or the air.
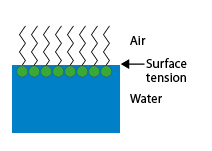
When this interface is the free surface of the solution in contact with the air, a surface film of oriented molecules is formed, which modifies its properties by reducing the surface tension, hence the name sometimes given to these products: “surface active agents”.
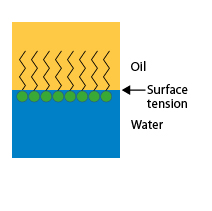
When the interface is the contact surface between two liquids, such as oil and water, surfactant molecules gather at this interface. Since the hydrophilic part of their molecule is in water and the hydrophobic part in oil, these molecules create a bond between the two liquids, which causes a decrease in surface tension and facilitates the formation of emulsions.
When the surfactant concentration of the aqueous solution is sufficient to saturate the interfaces, the excess molecules gather within the solution in small aggregates of oriented molecules, with the hydrophilic parts directed to the water and the hydrophobic parts directed towards the inside; these agglomerates are called micelles.
Focus on the structure of surfactants
Some products have a natural affinity for aqueous phases: sugars, glycerol, glycols, etc. These products are referred to as hydrophilic.
Other products have a natural affinity for oily phases: vegetable oils, paraffins, etc. These are referred to as hydrophobic or lipophilic.
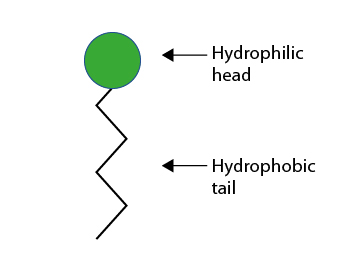
Products whose molecule includes a hydrophilic part and a hydrophobic part are amphiphiles. This is the case for surfactants represented by this symbol:
Four types of surfactants for
an endless scope of innovation!